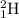Answer: Atoms of the same element have similar chemical behavior
Atoms of the same element can have different numbers of neutrons.
Step-by-step explanation:
Atoms of the same element have similar chemical behavior as it is decided by the number of electrons which remains same for same elements.
Mass number is defined as the sum of number of protons and neutrons that are present in an atom. Atomic number is defined as the number of protons or number of electrons that are present in an atom.
Isotopes are elements which have same atomic number but different mass number. That means they are same elements with same number of protons and electrons but different number of neutrons. Example:
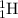 and
and