Answer:
1. This reaction is: B. Endothermic.
2. When the temperature is increased the equilibrium constant, K: A. Increases.
3. When the temperature is increased the equilibrium concentration of NO2: A. Increases.
Step-by-step explanation:
Hello,
In this case, considering the images, we can state that the red color at high temperature is due to the presence of nitrogen dioxide (product) and the lower coloring is due to the presence of dinitrogen tetroxide (reactant) at low temperature.
With the aforementioned, we can conclude that the chemical reaction:
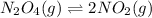
Is endothermic since high temperatures favor the formation of the product and the low temperatures favor the consumption of the the reactant. thereby:
1. This reaction is: B. Endothermic.
2. When the temperature is increased the equilibrium constant, K: A. Increases. In this particular case, since the dinitrogen tetroxide has 1 molecule and nitrogen dioxide two molecules in the chemical reaction, the entropy change should be positive, therefore, by increasing the T, the Gibbs free energy of reaction becomes more negative:
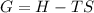
As Gibbs free energy becomes more negative, the equilibrium constant becomes bigger given their relationship:
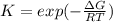
3. When the temperature is increased the equilibrium concentration of NO2: A. Increases.
Regards.