Answer:
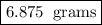
Explanation:
Half-lives are how long it takes for half of a substance to decay. If you start with a certain amount and it decays for a certain amount of half-lives, you are dividing by
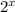 , where x is equal to the amount of half-lives.
, where x is equal to the amount of half-lives.
In order to solve this question, simply divide the starting value by 2 raised to the value of half-lives.
