Answer:
The correct option is;
a. 12.5 g
Step-by-step explanation:
The given parameters are;
The initial mass of the radioactive substance = 200 g
The half life of the radioactive substance = 50 years
The time duration for the disintegration = 200 years
The formula for half life is given as follows;
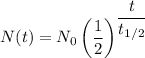
Where:
N(t) = Quantity of the remaining substance
N₀ = Initial radioactive substance quantity = 200 g
t = Time duration = 200 years
 = Half life of the radioactive substance 50 years
= Half life of the radioactive substance 50 years
Therefore, we have;
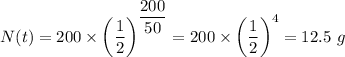
Therefore, at the end of 200 years, the quantity left = 12.5 g.