Answer:
B. 4.52 X10-9 M
Step-by-step explanation:
Our goal for this question is to calculate the concentration of hydronium ions
 produced by water in a vessel with a concentration of hydroxide ions of
produced by water in a vessel with a concentration of hydroxide ions of
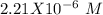 . So, our first approach can be the ionization reaction of water:
. So, our first approach can be the ionization reaction of water:
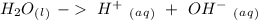
If we write the Keq expression for this reaction we will have:
![Keq=[H^+][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/9kmsf2i6h6xefqtqp14wv05uqv5qbw94sf.png)
Now, water is the universal solvent, so, Keq has a special name. In the equilibrium problems for water we have to use "Kw" instead of "Keq":
![Kw=[H^+][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/lnbme1jmg83pjr32ww4go5jfrufvmbk3ay.png)
From this equation, we know the Kw value () and the concentration of the hydroxide ions ([2.21X10^-^6~M]). If we replace these values into the equation we can solve for
![[H^+]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/rjqz56ud9q34ms188mnkhp8tjhwy4eib74.png) :
:
![1.0X10^-^1^4=[H^+][2.21X10^-^6~M]](https://img.qammunity.org/2021/formulas/chemistry/college/djaee07895s2w7nnccs7ty8j0g7zj4xmzk.png)
![[H^+]=(1.0X10^-^1^4)/(2.21X10^-^6)=4.52X^-^9](https://img.qammunity.org/2021/formulas/chemistry/college/mpmdsdza24mmrbcd5f59yl6akw62lke08v.png)
I hope it helps!