Answer:
- Addition of Ba(OH)2: favors the formation of a precipitate.
- Undergo a chemical reaction forming soluble species.
- Addition of CuSO4 : favors the formation of a precipitate.
Step-by-step explanation:
Hello,
In this case, since the dissociation reaction of barium sulfate is:
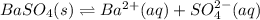
We must analyze the effect of the common ion:
- By adding barium hydroxide, more barium ions will be added to the equilibrium system so the formation of solid barium sulfate will be favored (reaction shifts leftwards towards reactants).
- By adding sodium nitrate, the following reaction will undergo:
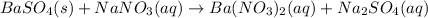
So the precipitate will turn into other soluble species.
- By adding copper (II) sulfate, more sulfate ions will be added to the equilibrium system so the formation of solid barium sulfate will be favored (reaction shifts leftwards towards reactants).
All of this is supported by the Le Chatelier's principle.
Best regards.