Answer:
a) 360.7 J/s
b) 16.23 °C
c) 34.48 g
Step-by-step explanation:
The mass of the person = 80 kg
The person is a perfect emitter, ε = 1
surface area of the person = 2.5 m^2
a) If he emits radiation at 37 °C,
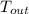 = 37 + 273 = 310 K
= 37 + 273 = 310 K
and receives radiation at 13 °C,
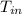 = 13 + 273 = 286 K
= 13 + 273 = 286 K
Rate of energy loss E = Aεσ(
 -
-
 )
)
where σ = 5.67 x 10^-8 J/(s m^2 K^4)
substituting values, we have
E = 2.5 x 1 x 5.67 x 10^-8 x (
 -
-
 ) = 360.7 J/s
) = 360.7 J/s
b) If they have specific heat about equal to that of water = 1 Cal/kg-°C
but 1 Cal = 1 kcal = 10^3 cal
specific heat of person is therefore = 10^3 cal/kg-°C
heat loss = 360.7 J/s = 360.7 x 3600 = 1298520 J/hr
heat lost in 1 hour = 1 x 1298520 = 1298520 J
This heat lost = mcΔT
where ΔT is the temperature fall
m is the mass
c is the specific heat equivalent to that of water
the specific heat is then = 10^3 cal/kg-°C
equating, we have
1298520 = 80 x 10^3 x ΔT
1298520 = 80000ΔT
ΔT = 1298520/80000 = 16.23 °C
c) 1298520 J = 1298520/4184 = 310.35 Cal
density of fat = 9 Cal/g
gram of fat = 310.35/9 = 34.48 g