Answer:
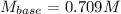
Step-by-step explanation:
Hello,
In this case, since the reaction between potassium hydroxide and nitric acid is:
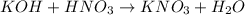
We can see a 1:1 mole ratio between the acid and base, therefore, for the titration analysis, we find the following equality at the equivalence point:

That in terms of molarities and volumes is:
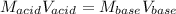
Thus, solving the molarity of the base (KOH), we obtain:
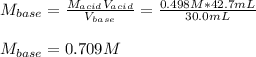
Regards.