Answer:
( a ) : Pool B
( b ) The final equilibrium temperature will be about 38°C
Step-by-step explanation:
Attachment 1 : The second pool ( pool B ) had more heat transferred to it, as you can see that it's temperature rose 10 degrees Celcius comparative to pool A, that rose by 9 degrees Celcius. Though the temperature difference is minumum, pool B did have more heat transferred to it.
Attachment 2 : So as we can see, the specific heat of iron and water is given by the units J / kg
 C. The mass of each substance is also given by kilograms, so there is no need for conversions here. The heat gained by each substance is equivalent to each other, so to determine the final temperature, consider the change in temperature in the formula q = m
C. The mass of each substance is also given by kilograms, so there is no need for conversions here. The heat gained by each substance is equivalent to each other, so to determine the final temperature, consider the change in temperature in the formula q = m
 c
c
 Δ T.
Δ T.
For the iron horseshoe, the change in temperature will be as such : Δ T =
 - 693, where
- 693, where
 = Final Temperature. Respectively the change in temperature for the water will be Δ T =
= Final Temperature. Respectively the change in temperature for the water will be Δ T =
 - 39, where
- 39, where
 = Final Temperature. Using this information let us solve for
= Final Temperature. Using this information let us solve for
 .
.
q = m
 c
c
 Δ T - where q = heat gained, m = mass of substance, c = specific heat of substance
Δ T - where q = heat gained, m = mass of substance, c = specific heat of substance
Δ T =
 - 693 and Δ T =
- 693 and Δ T =
 - 39,
- 39,
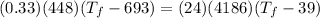 - let's say
- let's say
 = x,
= x,
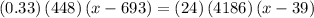 ,
,
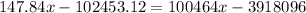 ,
,
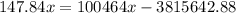 ,
,
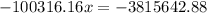 ,
,
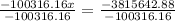 ,
,
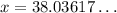
The final equilibrium temperature will be about 38°C.