Answer:
0.2 moles, assuming weight of dried salt
Step-by-step explanation:
In order to determine the number of moles, we need to be aware of the mass of the substance in question.
Assuming the mass of the dehydrated
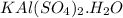 is 50g.
is 50g.
No. of moles = mass of substance/ molar mass of the substance.
=
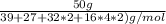
= 0.2 moles moles.