Answer: The mass of sodium chloride in 219 g solution is 32.9 g
Step-by-step explanation:
To calculate the mass percent of element in a given compound, we use the formula:
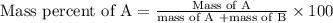
To find mass of sodium chloride in solution:
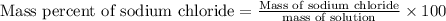
Mass percent of sodium chloride= 15.0 %
Mass of solution = 219g
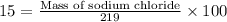
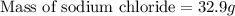
Thus mass of sodium chloride in 219 g solution is 32.9 g