Answer:
16.83°
Step-by-step explanation:
The computation of temperature in degrees is shown below:
For this we need to applied the combination of the Clasius Clapyeron equation which is
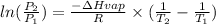
Where
P_2 and P_1 represents the pressure of vapor at point 1 and 2
dH = Here we use J/mol
R = 8.314 J/mol K
T1 and T2 represent the temperature of Saturation temperature for point 1 and 2
Now
we have to change the negative signs
i.e
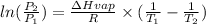
As we know that
dHvap for water = 40.67 kJ/mol
T1 = 373 K,
T2 = x
P1 = 14.7 psi;
P2 = 11.2 + 14.7 = 25.9 psi
Now put the values to the above formula
So,
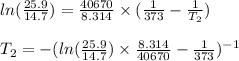
After solving this
T_2 = 389.836K
T_2 = 389.836 - 273
T_2 = 116.836 °C
Therefore by 16.83°, it becomes higher