Answer: The given statement is TRUE.
Step-by-step explanation:
An equilibrium reaction is one in which rate of forward reaction is equal to the rate of backward reaction.
Equilibrium constant is defined as the ratio of the product of the concentration of products to the product of the concentration of reactants each raised to their stochiometric coefficient.
For example for the given equilibrium reaction;
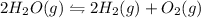
![K_(eq)=([H_2]^2[O_2])/([H_2O]^2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/ato1x78e0nbhzusf1os332xbm2huv033kj.png)
Thus the given statement that in calculating the equilibrium constant for a reaction, the coefficients of the chemical equation are used as exponents for the factors in the equilibrium expression is True.