Answer:
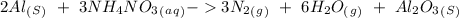
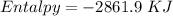
Step-by-step explanation:
In this case, we have to start with the reagents:
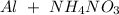
The compounds given by the problem are:
-) Nitrogen gas =

-) Water vapor =

-) Aluminum oxide =
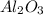
Now, we can put the products in the reaction:
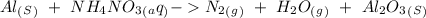
When we balance the reaction we will obtain:
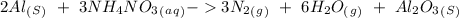
Now, for the enthalpy change, we have to find the standard enthalpy values:
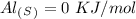
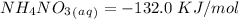
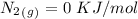
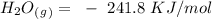
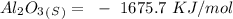
With this in mind, if we multiply the number of moles (in the balanced reaction) by the standard enthalpy value, we can calculate the energy of the reagents:

And the products:
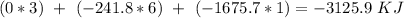
Finally, for the total enthalpy we have to subtract products by reagents :
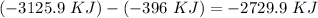
I hope it helps!