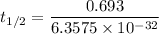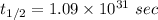Answer:
Step-by-step explanation:
GIven that:
The activation energy = 250 kJ
k₁ = 0.380 /M
k₂ = ???
Initial temperature
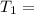 1001 K
1001 K
Final temperature
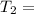 298 K
298 K
Applying the equation of Arrhenius theory.
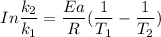
where ;
R gas constant = 8.314 J/K/mol
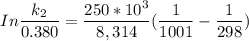
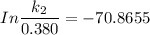
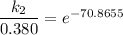
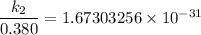
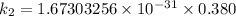
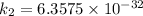 /M .sec
/M .sec
Half life:
At 1001 K.
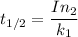
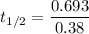
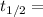 1.82368 secc
1.82368 secc
At 298 K: