Answer:
3 grams will be left after 6 half-lives
Explanation:
Half-live:
Time it takes for the substance to be reduced by hall.
After n half lives:
The amount remaing is:
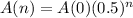
In which A(0) is the initial amount and n is the number of half-lives.
Starting with 210 grams of a radioactive isotope, how much will be left after 6 half-lives?
This is A(6) when A(0) = 210. So
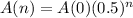
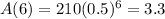
Rounding to the nearest gram
3 grams will be left after 6 half-lives