Answer:
E = 18,600 J
Step-by-step explanation:
It is given that,
Number of moles = 3
Temperature, T = 25°C = 25+273 = 298 K
The internal energy of N₂ gas is given by :
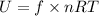
f is degrees of freedom. For diatomic gas, degree of freedom is equal to 5/2. So,
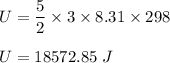
or
U = 18600 J
So, the internal energy of the gas is 18,600 J