Answer:
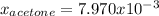
Step-by-step explanation:
Hello,
In this case, for the given molarity, we can assume a volume of 1 L of solution, to obtain the following moles of acetone:
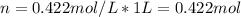
Then, with the density of solution, we can compute the mass of the solution for the selected 1-L volume basis:
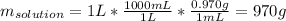
After that, we compute the mass of water in the solution, considering the mass of acetone (molar mass = 58.08 g/mol):
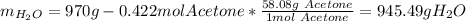
Next, the moles of water:
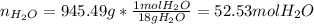
Finally, the mole fraction:
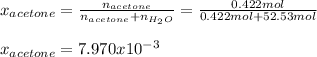
Regards.