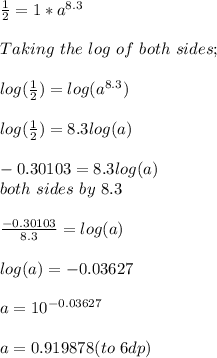Answer:
Explanation:
Given the half like of a material to be 8.3 hours and the amount of iron-52 left after t hours is modeled by the equation
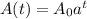 , we can get A(t) as shown;
, we can get A(t) as shown;
At t = 8.3 hours, A(8.3) = 1/2
Initially at t = 0; A(0) = 1
Substituting this values into the function we will have;