Answer:
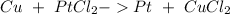
Step-by-step explanation:
In this case, we can start with the formula of Platinum (II) Chloride. The cation is the atom at the left of the name (in this case
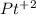 ) and the anion is the atom at the right of the name (in this case
) and the anion is the atom at the right of the name (in this case
 ). With this in mind, the formula would be
). With this in mind, the formula would be
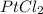 .
.
Now, if we used metallic copper we have to put in the reaction only the copper atom symbol
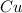 . So, we have as reagents:
. So, we have as reagents:
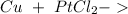
The question now is: What would be the products? To answer this, we have to remember "single displacement reactions". With a general reaction:
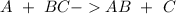
With this in mind, the reaction would be:
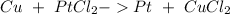
I hope it helps!