Answer:
Following are the answer to this question:
Step-by-step explanation:
The value of pH solution is =5.17 So, the p^{OH}:
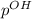 =14-56.17
=14-56.17
=8.823
The volume of the
 = 40.00 ml
= 40.00 ml
convert into the liter= 0.040L
The value of the concentrated
 =0.10 M
=0.10 M
The volume of the
 = 50.00 ml
= 50.00 ml
convert into the liter= 0.050L
The value of concentrated
 = 0.10 M
= 0.10 M
The volume of the
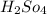 = 30 ml
= 30 ml
convert into the liter= 0.030L
The value of concentrated
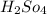 =0.05 M
=0.05 M
Calculating total volume=(0.40+0.050+0.030)
=0.120 L
calculating the new concentrated value of
 =
=
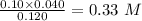
calculating the new concentrated value of
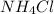 =
=
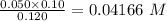 calculating the new concentrated value of
calculating the new concentrated value of
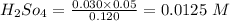 when 1 mol
when 1 mol
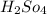 produced 2 mols
produced 2 mols
 so, 0.0125 in
so, 0.0125 in
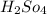 produced:
produced:
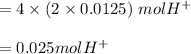
create the ICE table:
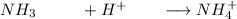
I (m) 0.033(m) 0.025 0.04166
C -0.025 -0.025 + 0.025
E 8.3\times 10^{-3} 0 0.0667
now calculating pH:
when ph= 8.83:
![P^(H)= p^(kb)|+ \log([NH_4^(+)])/([NH_3])\\\\8.83=p^(kb)+\log(0.0667)/(8.3 * 10^(-3))\\\\p^(kb)=8.83-0.9069\\\\ \ \ \ =7.7231 \\\\\ The P^(kb) \ for \ NH_3 \ is =7.7231\\\\\ The P^(kb) \ for N^(+)H_4=14-7.7231\\\\\ \ \ \ \ \ =6.2769](https://img.qammunity.org/2021/formulas/chemistry/college/21ontvded574nhol6zscxh8k7dkr350dz5.png)