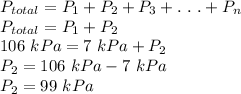Answer:
b) 99 kPa
Step-by-step explanation:
According to Daltons law of partial pressure, the total pressure of a mixture of two or more non reactive gases is the sum of their individual pressures. Let the total pressure of a mixture of n number of gases be
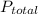 and their individual pressure be
and their individual pressure be
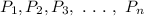 , According to Daltons partial pressure law:
, According to Daltons partial pressure law:
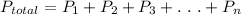
Since A glass cylinder contains 2 gases at a pressure of 106 kPa, therefore n = 2. Also one gas (
 ) is at 7 kPa. Using Daltons partial pressure law:
) is at 7 kPa. Using Daltons partial pressure law: