Answer:
The number of oxide ions as the nearest neighbors of
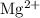 ions are known to be as six
ions are known to be as six
Step-by-step explanation:
The regularity of a crystal structure leads to the idea of space lattice.In order to explain this concept, let us consider a crystal of NaCl, It consists of a perfectly regular arrangement of sodium ions and chlorine ions.
If we represent the position of each Na+ in the crystal by a point marked x the result will be a regular three dimensional network of points. This will be the space lattice of Na+ in the crystal NaCl. The symmetry of the combined lattice determined the symmetry of the crystal as a whole.
The space lattice of a crystal may be considered as built up of a three dimensional basic pattern called unit cell. The unit cell is a repeat unit which generates the whole pattern in three dimensions of the unit cell.
In Solid MgO , the crystal structure which is used to predict the properties of the material, have the same structure as that of NaCl.
The obtain the structure of a face centered cubic FCC unit cell where the ions occupy the corner of the cube and the center of each face of the cube.
The number of oxide ions as the nearest neighbors of
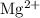 ions are known to be as six. As a result of that , the coordination number of
ions are known to be as six. As a result of that , the coordination number of
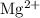 ions is six.
ions is six.