Answer:
1.076 mol (corrected to 2 d.p.)
Step-by-step explanation:
Take the atomic mass of Ga be 69.7.
since no. of moles = mass/ molar mass,
no. of moles of Ga used = 100.0 / 69.7
= 1.43472023 mol
From the balanced equation, the mole ratio of Ga:S2 = 4:3, which means every 4 moles of Ga can react completely with 3 moles of S2.
So, let the no. of moles of Sulphur required be y.
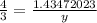
4 y = 1.43472023 x 3
y = 1.076 mol (corrected to 2 d.p.)