Answer:
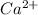
Step-by-step explanation:
From the periodic table, we can see that the atom with 20 protons is Calcium (Ca).
Each proton has a +1 charge, while each electron has a -1 charge.
Since in this ion, there's 20 protons but only 18 electrons, meaning it has a net charge of +2.
Hence, the ion symbol for the required proton is
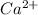 .
.
(make sure the charge symbol is behind the value.)