Plz help!!!
some iron filings were shaken with some copper (II) sulfate solution. The ionic equation for the reaction is :
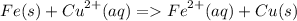
a)write down any one change that you would observed during this reaction
b)which substance has been oxidized in this reaction
c) write down the full (not ionic) equation for this