Answer:
Explanation:
The formula you will need for this is:
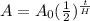 where A is the amount after some decay has happened, A₀ is the intial amount, t is the time in hours, and H is the half-life in hours. Those values for us are:
where A is the amount after some decay has happened, A₀ is the intial amount, t is the time in hours, and H is the half-life in hours. Those values for us are:
A = 2 g
A₀ = 25 g
H = 4.95 hrs and
t = ?
Filling in:
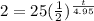 Keep in mind that, because of the nature of the exponential form of this equation, you CANNOT simply multiply the 25 by the 1/2. Exponential equations don't work that way. Begin instead by dividing both sides by 25 to get
Keep in mind that, because of the nature of the exponential form of this equation, you CANNOT simply multiply the 25 by the 1/2. Exponential equations don't work that way. Begin instead by dividing both sides by 25 to get
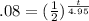 The goal is to get that t out from its exponential position. Do that by taking the natural log of both sides:
The goal is to get that t out from its exponential position. Do that by taking the natural log of both sides:
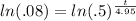
After you take the natural log of the right side, the property allows you to bring the exponent down out front:
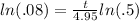
Now divide both sides by ln(.5) to get
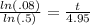
Simplify the left side out on your calculator to get
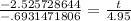 and then divide:
and then divide:
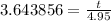
Finally, multiply both sides by 4.95 to get
3.643856(4.95) = t so
t = 18.0 hours which is choice C