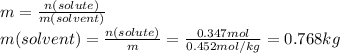Answer:
0.768 kg
Step-by-step explanation:
Step 1: Given data
- Mass of ammonium nitrate (solute): 27.8 g
- Molality of the solution (m): 0.452 m
Step 2: Calculate the moles corresponding to 27.8 g of ammonium nitrate
The molar mass of ammonium nitrate is 80.04 g/mol.
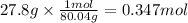
Step 3: Calculate the mass of water
The molality is equal to the moles of solute divided by the kilograms of solvent (water).