Answer:
80 kcal
Step-by-step explanation:
The computation of the kilocalories of heat required is shown below:
As we know that
1 liter of water = 1 kg
Therefore the specific heat of water is C = 1 kcal per kg
Now the heat required is
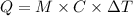
where,
M = 1
C = 1
Delta T = (100 -20)
As we assume that boiling point of water is 100 degrees
Now put this values to the above formula
So, the heat required is
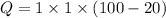
= 80 kcal