Answer:
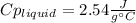
Step-by-step explanation:
Hello,
In this case, since silver is initially hot as it cools down, the heat it loses is gained by the liquid, which can be thermodynamically represented by:
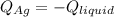
That in terms of the heat capacities, masses and temperature changes turns out:
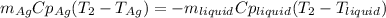
Since no phase change is happening. Thus, solving for the heat capacity of the liquid we obtain:
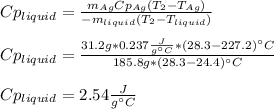
Best regards.