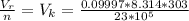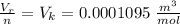Answer:
The ideal molar volume is
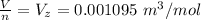
The Z factor is
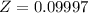
The real molar volume is
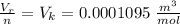
Step-by-step explanation:
From the question we are told that
The pressure is
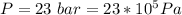
The temperature is
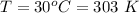
According to the ideal gas equation we have that
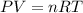
=>
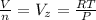
Where
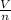 is the molar volume and R is the gas constant with value
is the molar volume and R is the gas constant with value
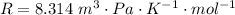
substituting values
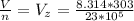
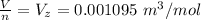
The compressibility factor of the gas is mathematically represented as
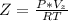
substituting values
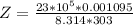
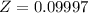
Now the real molar volume is evaluated as
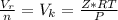
substituting values