Answer:
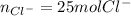
Step-by-step explanation:
Hello,
In this case, since the given 5-M concentration of magnesium chloride is expressed as:
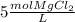
We can notice that one mole of salt contains two moles of chloride ions as the subscript of chlorine is two, in such a way, with the volume of solution we obtain the moles of chloride ions as shown below:
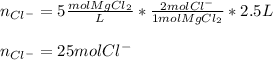
Best regards.