Answer:
0.901 M
Step-by-step explanation:
The concentration of the CO(g) in the equilibrated mixture is shown below:
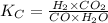
where,
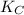 = equilibrium constant
= equilibrium constant
And, we placing these above values to the formula shown as above
So, the concentration of CO(g) is
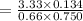
=
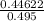
= 0.901 M
We simply applied the above formula in order to determine it and so that the correct answer could arrive