Answer:
0.01958mol
Explanation:
you want to go from grams to mols so when set up it looks like this
6.7 x
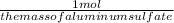
(you want what your multiplying to cancel out with the denominator(g cancel out g so your left with mols))
to get the mass of aluminum sulfate you must first find the chemical formula which is
Al2(SO4)3
get the amount of every element
so there is:
2 Al
3 S
12 O
(multiply the three to everything inside the parenthesis)
next find the total mass of everything
Al=26.982g x 2
S=32.06g x 3
O=16g x 12
add everything together and you get 342.144g so you plug that number into the first equation
6.7 x
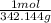 →
→
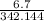 →0.01958mol
→0.01958mol