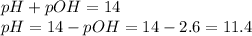Answer:
11.4
Step-by-step explanation:
Step 1: Given data
- Concentration of the base (Cb): 0.300 M
- Basic dissociation constant (Kb): 1.8 × 10⁻⁵
Step 2: Write the dissociation equation
NH₃(aq) + H₂O(l) ⇄ NH₄⁺(aq) + OH⁻(aq)
Step 3: Calculate the concentration of OH⁻
We will use the following expression.
![[OH^(-) ]=√(Kb * Cb ) = \sqrt{1.8 * 10^(-5) * 0.300 } = 2.3 * 10^(-3) M](https://img.qammunity.org/2021/formulas/chemistry/college/4zpokf4j3b0vyjcyyffd6b42ykn7sjx1qw.png)
Step 4: Calculate the pOH
We will use the following expression.
![pOH =-log[OH^(-) ]= -log(2.3 * 10^(-3) M) = 2.6](https://img.qammunity.org/2021/formulas/chemistry/college/gfwwf38vdzfvvfe8m91ajguasyf92n0f6b.png)
Step 5: Calculate the pH
We will use the following expression.