Answer:
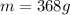
Step-by-step explanation:
Hello,
In this case, in order to compute the required mass, we first must notice that 6.022x10²³ formula units of sodium sulfate contain 1 mole of such compound (Avogadro's relationship). Moreover, one mole of sodium sulfate contains 142.04 g, which is in fact, the molar mass. Thereby, the required mass is computed via the following mole-mass-particles relationship:
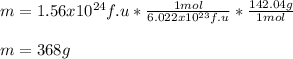
Regards.