Answer:
9.0 × 10⁻⁴ mol of K⁺
3.0 × 10⁻⁴ mol of PO₄³⁻
Step-by-step explanation:
Step 1: Calculate the moles of potassium phosphate in 20.0 mL of a 0.015 M solution
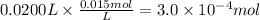
Step 2: Write the balanced dissociation reaction
K₃PO₄(aq) ⇒ 3 K⁺(aq) + PO₄³⁻(aq)
Step 3: Calculate the moles of K⁺
The molar ratio of K₃PO₄ to K⁺ is 1:3. The moles of K⁺ are 3/1 × 3.0 × 10⁻⁴ mol = 9.0 × 10⁻⁴ mol
Step 4: Calculate the moles of PO₄³⁻
The molar ratio of K₃PO₄ to PO₄³⁻ is 1:1. The moles of PO₄³⁻ are 1/1 × 3.0 × 10⁻⁴ mol = 3.0 × 10⁻⁴ mol