Answer:
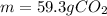
Step-by-step explanation:
Hello,
In this case, the first step is to compute the molar mass of carbon dioxide as shown below, considering it has one carbon atom and two oxygen atoms:
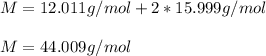
It is important to notice it is the mass in one mole of such compound. Afterwards, we need to use the Avogadro's number to compute the how many moles are in the given molecules of carbon dioxide as shown below:
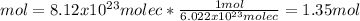
Finally, the mass by using the molar mass:
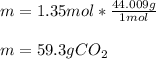
Best regards.