Answer:
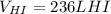
Step-by-step explanation:
Hello,
In this case, for the given balanced chemical reaction:
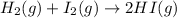
Thus, since hydrogen and hydrogen iodide are in a 1:2 mole ratio, we can easily compute the yielded volume as shown below:
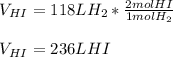
Thus, is possible, due to the Avogadro's law which allows to relate moles and volume by a directly proportional relationship.
Regards.