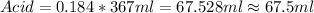Answer:
For this case we know that the total amount of solution including water and acid is V=367 ml
And we know that the % of acid in the solution is 18.4% so then we can find the number of ml of acid witht this operation:
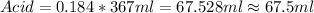
Explanation:
For this case we know that the total amount of solution including water and acid is V=367 ml
And we know that the % of acid in the solution is 18.4% so then we can find the number of ml of acid witht this operation: