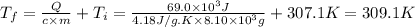Answer:
309.1K
Step-by-step explanation:
Step 1: Convert the flown heat to Joule
We will use the relationship 1 kJ = 1,000 J.
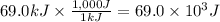
Step 2: Convert the mass of water to gram
We will use the relationship 1 kg = 1,000 g.
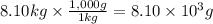
Step 3: Convert the initial temperature to Kelvin
We will use the following expression.
K = °C + 273.15 = 33.9°C + 273.15 = 307.1 K
Step 4: Calculate the final temperature
We will use the following expression.
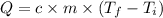
where,
- c: specific heat capacity