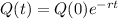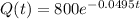Answer:
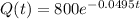
Explanation:
The amount of the isotope after t seconds is given by the following exponential equation:
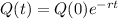
In which Q(0) is the initial amount and r is the decay rate.
A sample of beryllium-11 was first measured to have 800 atoms.
This means that
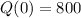
The half-life of the isotope beryllium-11 is 14 seconds.
This means that
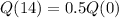
We use this to find r.
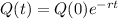
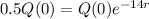
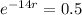
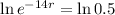
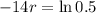
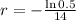
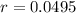
So