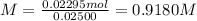Answer:
0.9180 M
Step-by-step explanation:
Step 1: Write the balanced equation
H₂SO₄(aq) + 2 KOH(aq) ⇒ K₂SO₄(aq) + 2 H₂O(l)
Step 2: Calculate the reacting moles of KOH
27.00 mL of 1.700 M KOH react. The reacting moles of KOH are:
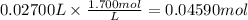
Step 3: Calculate the reacting moles of H₂SO₄
The molar ratio of H₂SO₄ to KOH is 1:2. The reacting moles of H₂SO₄ are 1/2 × 0.04590 mol = 0.02295 mol.
Step 4: Calculate the molarity of H₂SO₄
0.02295 moles of H₂SO₄ are in 25.00 mL of solution. The molarity of the acid solution is: