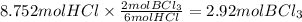Answer:
2.92 mol
Step-by-step explanation:
Step 1: Write the balanced equation
2 B(s) + 6 HCI(aq) ⇒ 2 BCl₃(aq) + 3 H₂(g)
Step 2: Establish the appropriate molar ratio
The molar ratio of hydrochloric acid to boron chloride is 6:2.
Step 3: Calculate the moles of boron chloride produced from 8.752 moles of hydrochloric acid