Answer:
Theoretical maximum moles of hydroquinone: 0.2167 mol.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is like:
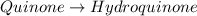
In such a way, since the molar mass of quinone is 108.1 g/mol and it is in a 1:1 molar ratio with hydroquinone, we can easily compute the theoretical maximum moles of hydroquinone by stoichiometry:
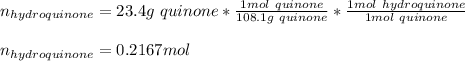
Clearly, this is the theoretical yield which in grams is:
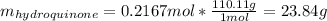
Which allows us to compute the percent yield as well since the obtained mass of the product is 13.0 g:
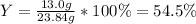
Best regards.