Answer:
number of moles of the compound
 53 mole
53 mole
Step-by-step explanation:
Given that:
The total energy liberated = - 2870 kJ ( here , the negative sign typical implies the release of energy due to the combustion reaction)
The equation of the reaction can be represented as:
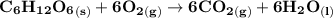
The energy needed to synthesize 1 mole of compound X = - 54.1 kJ.mol
Thus;
The total energy = numbers of moles of compound × Energy needed to synthesize 1 mole of compound X
Making the numbers of moles of the compound the subject; we have;
numbers of moles of compound =
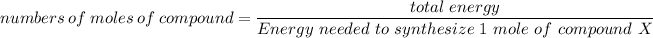
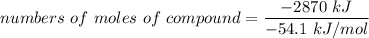
number of moles of the compound = 53.04990 mole
number of moles of the compound
 53 mole to two significant figure
53 mole to two significant figure