Answer:
Approximately
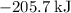 .
.
Step-by-step explanation:
This question can be solved using Hess's Law.
Start by considering: how can the first three reactions (with known
 values) be combined to produce the reaction
values) be combined to produce the reaction
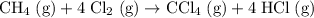 ?
?
Here's one possible combination:
- Include the first reaction once, without inverting.
- Invert the second reaction and include it once.
- Include the third reaction after multiplying all its coefficients by two.
In other words, if
 ,
,
 , and
, and
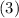 denote the three reactions with know
denote the three reactions with know
 values, respectively, then
values, respectively, then
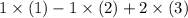 will give the required reaction
will give the required reaction
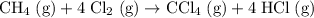 .
.
By Hess's Law, the
 value of the reaction
value of the reaction
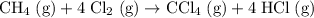 will thus be:
will thus be:
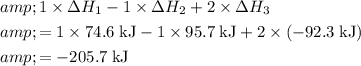 .
.