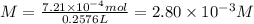Answer:
2.80 × 10⁻³ M
Step-by-step explanation:
There is some info missing. I think this is the original question.
One cup of fresh orange juice contains 127 mg of ascorbic acid (vitamin C, C₆H₈O₆). Given that one cup = 257.6 mL, calculate the molarity of vitamin C in orange juice.
Express your answer to an appropriate number of significant figures with the appropriate units.
Step 1: Convert the mass of ascorbic acid to grams
We will use the relationship 1 g = 1,000 mg.
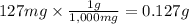
Step 2: Calculate the moles corresponding to 0.127 g of ascorbic acid
The molar mass of ascorbic acid is 176.13 g/mol.
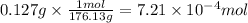
Step 3: Convert the volume of the solution to liters
We will use the relationship 1 L = 1,000 mL.
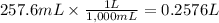
Step 4: Calculate the molarity of the solution