Answer:
ΔQ = 0.1 kJ
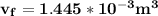
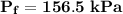
ΔS = -0.337 J/K
The value negative is due to the fact that there is need to be the same amount of positive change in surrounding as a result of compression.
Step-by-step explanation:
GIven that:
Diameter of the piston-cylinder = 12 cm
Pressure of the piston-cylinder = 100 kPa
Temperature =24 °C
Length of the piston = 20 cm
Boundary work ΔW = 0.1 kJ
The gas is compressed and The temperature of the gas remains constant during this process.
We are to find ;
a. How much heat was transferred to/from the gas?
According to the first law of thermodynamics ;
ΔQ = ΔU + ΔW
Given that the temperature of the gas remains constant during this process; the isothermal process at this condition ΔU = 0.
Now
ΔQ = ΔU + ΔW
ΔQ = 0 + 0.1 kJ
ΔQ = 0.1 kJ
Thus; the amount of heat that was transferred to/from the gas is : 0.1 kJ
b. What is the final volume and pressure in the cylinder?
In an isothermal process;
Workdone W =
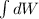
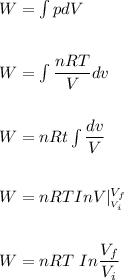
Since the gas is compressed ; then
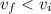
However;
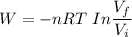
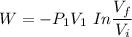
The initial volume for the cylinder is calculated as ;
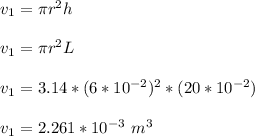
Replacing over values into the above equation; we have :
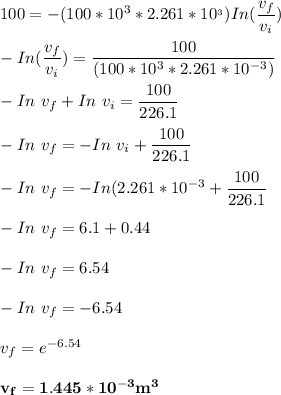
The final pressure can be calculated by using :
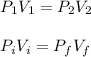
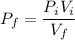
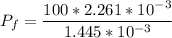
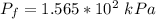
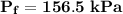
c. Find the change in entropy of the gas. Why is this value negative if entropy always increases in actual processes?
The change in entropy of the gas is given by the formula:
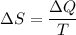
where
T = 24 °C = (24+273)K
T = 297 K
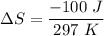
ΔS = -0.337 J/K
The value negative is due to the fact that there is need to be the same amount of positive change in surrounding as a result of compression.