Answer:
2.22 g/L
Step-by-step explanation:
There's a relationship using the ideal gas law between molar mass and density:
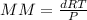 , where MM is the molar mass, d is the density, R is the gas constant, T is the temperature, and P is the pressure.
, where MM is the molar mass, d is the density, R is the gas constant, T is the temperature, and P is the pressure.
We know from the problem that MM = 32.49 g/mol, T = 458 Kelvin, and P = 2.569 atm. The gas constant, R, in terms of the units atm and Kelvin is 0.08206. Let's substitute these values into the formula:
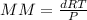
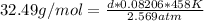
Solve for d:
d * 0.08206 * 458 K = 32.49 * 2.569
d = (32.49 * 2.569) / (0.08206 * 458 K) ≈ 2.22 g/L
The answer is thus 2.22 g/L.
~ an aesthetics lover